|
Home
What is Half–Life?
The half-life of a radioactive element is the time taken for half its mass to decay. The half-life of an element is a constant value for the particular element, irrespective of the quantity of the element present.
Knowing the half-life of an element makes it possible to detect it and to calculate the quantity that had decayed, or the quantity left over a given time.
The rate at which radioactive disintegration occurs (i.e., half-life) vary from very slow to very rapid. For radium, the half-life is 1590 years.
This means that, on the average, half of the radium atoms contained in a particular sample
will disintegrate in 1590 years, etc. Half-life periods vary from fourteen billion years for one of the isotopes of thorium to less than one billionth of a second for one of the isotopes of polonium.
The longer the half-life of a substance, the weaker will be the intensity of its radiation.
Therefore, since in humans physiological effects result from exposures to radiations, such effects are milder from substances with longer half-life.
Example: A sample of radioactive element with half-life of 2 mins initially contains 8 x 106 atoms, find the time at which the number of atoms of the original element of the sample is 2 x 106 atoms.
Solution:
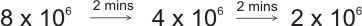
I.e., the substance went through two disintegrations, \ total time taken is
2+2 = 4 mins.
|