|
Home
Covalent Bonding
Covalent bonding
is a type of bonding that involves the participants contributing equal number of electrons into a shared pair.
The electrons in the shared pair revolve round the nuclei of the atoms involved, such that each atom assumes the electronic configuration of an inert gas and becomes electronically stable.
Notice that the bond is the shared pair between the participating atoms.
Covalent bonds are formed between atoms of the same element; and between atoms of elements at the middle and those at the extreme right of a period in the period table.
Here, actual molecules are formed and not ions.
Examples of Covalent Bonding
Molecules formed by covalent bonding include: Cl2, CH4, HCl, NH3, H2O and CH3CH2OH.
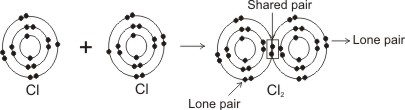
Covalent bonding in chlorine molecule, Cl2:
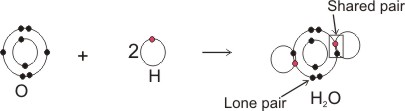
Covalent bonding in water molecule, H2O:
Covalent bonding in ethanol, CH3CH2OH:
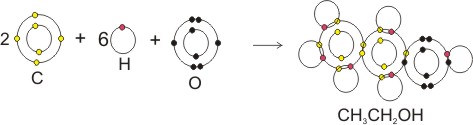
Note: each shared pair represents a bond. Each atom in a bond becomes isoelectronic with the inert or noble gases, thereby becoming stable.
Properties of Covalent Compounds
1. They are non-conductors of electricity.
2. Simple covalent compounds are gases or volatile liquids. This is because their molecules are electrically neutral and have little attractive forces for each other. More complex covalent molecules (e.g. naphthalene), which are crystalline, have van der waals forces of attraction between the molecules – this results in the crystals having low melting points (81oC).
3. They are usually soluble in covalent organic solvents, such as benzene and carbon
disulphide.
Covalent Bonding in Elements
The atoms in an element are held together by covalent bonding. The presence of this bonding explains the hardness, strength and melting points of elements.
The variation of the melting points of elements in the periodic table shows that in period 2, melting points gradually increase from Li (group IA), and reaches a maximum in C (grp IVA). It then starts decreasing from nitrogen to neon (group 0).
The trend begins again from sodium (group IA), gets to maximum in Si (group IVA), and begins to decrease again from P to Ar. The explanation of this trend is that the higher the valency of an element , the greater the degree of covalency between its atoms.
Thus making the element harder, and therefore of higher melting point. Carbon is tetravalent (i.e.,
valency of 4) – it has the greatest covalency, which is reinforced by the closeness of its valence electrons to the nucleus. In diamond (an allotrope of carbon), each atom is covalently held to four others, making a crystal unit. This crystal unit is repeated indefinitely, forming interlacing
buckled hexagons. The strength and uniformity of bonding makes diamond: resistant to melting; non-volatile; and the hardest natural substance.
|

