|
Home
Chloroform Hazards: Formula, Uses, Properties
This post provides exhaustive information about the chloroform, its formula, properties, and uses, and the hazards associates with it.
It provides useful ideas on how to handle chloroform and prevent exposure to the hazards associated with it.
Please, read on to learn about the chemical compound chloroform:
 What is Chloroform? What is Chloroform?
Chloroform is a colorless liquid chemical substance with a pleasant smell. It is an organic compound used in many labs and industries.
It is a harmless substance and very hazardous and toxic to health and the environment.
It has the ability to make an individual unconscious and can even lead to death; however, it is useful in medical facilities and even in industries.
Chloroform Formula
Chloroform has the chemical formula CHCI3. This shows three chlorine atoms and one hydrogen atom in a covalent bond with a carbon atom. Its IUPAC name is Trichloromethane.
Common Chloroform Hazards
Over the years, chloroform has caused so many menace environmentally and even health wise. Some of those common hazards include:
• Inhalation: Inhaling chloroform is very dangerous to health; it affects the heart so much that it no longer beat properly. Even the liver and lungs are not excluded from this chemical’s toxicity when inhaled. This incidence can put the victim’s life at stake. Extreme exposure to this chemical can also cause the victim to experience headache, blurry vision or dizziness, nausea, and vomiting. Ingesting chloroform is also deadly, it harms the gastrointestinal areas.
• Chloroform is also suspected to be one of the causes of various types of cancers.
Chloroform Safety Handling Procedures
There are some things you have do when handling this chemical in order to protect yourself properly.
Apply the following methods when using chloroform to prevent harm:
• Always cover up your body with protective clothing
• Make sure your hands are well covered with chemical proof gloves
• Your eyes are not excluded, protect them with goggles
• Clean up your environment after use, in case of spills on the floor and otherwise
• Always remember to take your bath after handling chloroform.
Chloroform Safety Storage Procedures
Always store chloroform in the proper place to prevent accidents. Follow this storage method to keep chloroform safe:
• Storing this chemical together with combustible items is very dangerous
• Always make sure it is separated far away from heat or fire
• Store chloroform in a tightly sealed container
• A cool and dry environment is a very good place to store chloroform.
Chloroform Safety Disposal Procedures
Chloroform seems to be harmless due to its natural pleasant smell, but it is very toxic to the environment whenever it is not disposed properly.
Disposing this chemical is not a practice that you can carry out all by yourself. You need to follow a special procedure that demands you to mix chloroform with combustible fuel to make it very safe for disposal.
Also, any hazardous waste facility that is close to your residence can be of help. They know the best way to dispose this chemical.
You should weigh in a number of things you need to consider when disposing this chemical. You don’t flush it down the drain, or pour it on the ground. It’s a very bad idea to do so.
How to Handle Exposures to Chloroform
Chloroform has done a lot of harm to people because of ignorance. There are first aid measures that should be applied whenever someone gets exposed to this chemical.
The common exposure to chloroform is inhalation (breathing it in). This is very deadly to health and can also result to death if it is inhaled for a long time.
If someone inhales this chemical, the victim should be taken to an open space that has enough fresh air. He/she should be spirited to the nearest medical facility for attention immediately.
If your skin is exposed to chloroform, take off your clothes (that has chloroform), then get a very good bath.
You can call for medical attention if you still feel unwell.
Chloroform can damage the eyes in no time if they get in, rinse your eyes with a special eyewash before consulting a medical facility.
Additional Chloroform Information
Here are additional facts about chloroform, including properties, preparation, and uses to help you learn more about it:
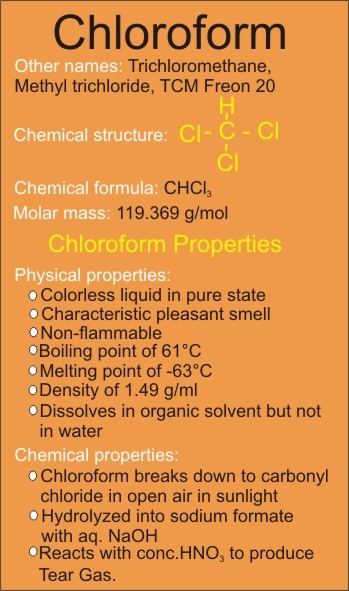
Chloroform Properties (Physical and Chemical)
Physical Properties
• Chloroform has a pleasant smell
• Non-flammable
• Molecular Weight of 119.369 g/mol
• Boils at 61 Celsius degree and melts at -63 oC
• Density of 1.49 g/cm³
• It dissolves in organic solvent but not in water
• Chloroform becomes phosgene when mixed with air.
Chemical Properties
1. Chloroform breaks down to carbonyl chloride when exposed to open air in the presence of sunlight.
2CHCl3 + O2 –Sunlight–> COCl2 + HCl
2. Chloroform gets hydrolyzed into sodium formate when the other reactant is aq. NaOH.
CHCl3 + aq.3NaOH –heat–> [H-C(OH)3] + H2O
Here [H-C(OH)3] later becomes methanoic acid since it is unstable leading to the reaction below
HCOOH + NaOH —-> HCOONa + H2O
Thus HCOONa is the last compound formed from this reaction.
3. Reaction with conc.HNO3
Tear Gas is produced when chloroform is heated with conc.HNO3
CHCl3 + conc.HNO3 –Over Heat–> CCl3NO2 + H2O
Chloroform Preparation
In industrial settings, chlorination of methane is the route to making chloroform. Additionally, on an industrial basis, when carbon tetrachloride undergoes partial reduction with iron filings and stream.
Chloroform Uses
Chloroform does not have so many uses, however, its uses include:
• To preserve specimens in the laboratory
• It is for extraction of DNA for analysis
• For extracting flavors, antibiotics, vitamins
• Used for producing fumigants, dyes, pesticides.
|