|
Home
What is Hydrogen Bonding?
Hydrogen Bonding
is a force which holds molecules of the same substance together. It is is found mainly in compounds in which hydrogen is covalently bonded to the highly electronegative elements – fluorine, oxygen, nitrogen, and to some extent chlorine.
Hydrogen bonding is a strong type of intermolecular force, as bonding is caused by electrostatic attraction of the highly positive hydrogen of one molecule to the highly negative fluorine, oxygen or nitrogen of another molecule - this is a special type of electrovalent bonding.
Examples of compounds which exhibit hydrogen bonding include HF, H2O, NH3, H2SO4, C2H5OH and HCOOH.
Explanation of the bonding:
The electronegative element in the compound exerts a greater pull on the shared electrons, thereby creating a partial negative charge on itself and a partial positive charge on the hydrogen. The positive hydrogen then forms a bond with the electronegative atom of a neighboring molecule, while its electronegative element forms another bond with positive hydrogen of another neighboring molecule.
Example (i).
 (bonding electrons drift more to the fluorine). (bonding electrons drift more to the fluorine).
Therefore, several molecules combine by hydrogen bonding thus:
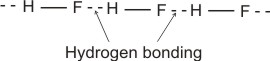
(ii). In H2O
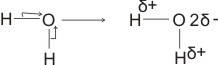
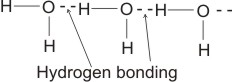
(bonding electrons drift more to the more electronegative oxygen).
Therefore, several molecules combine by hydrogen bonding thus:
Factors which Determine The strength of Hydrogen Bonding:
1. The electronegativity of the atom bonded to hydrogen - The greater the electronegativity of the atom bonded to hydrogen, the stronger the hydrogen bonding (e.g. hydrogen bonding in HF is greater than in HCl.
2. The number of possible hydrogen bonding per molecule.
Example: Hydrogen bonding is greater in H2O than in HF. This is because, even though fluorine is more electronegative than oxygen (the difference is not much), the number of hydrogen bonding that a molecule of water can form is 4 (i.e. two by the 2 lone pairs of electrons on the oxygen, and another two by each hydrogen), while in HF, the number of hydrogen bonding per molecule is 2 (one each by the hydrogen and fluorine atoms).
The following gives the order of strength of hydrogen bonding in the compounds:
H2O > HF > NH3 > HCl
Hydrogen bonding is stronger in NH3 than in HCl due to greater number of hydrogen bonding in NH3 than in HCl. Certain organic compounds, such as alkanols, e.g. ethanol (CH3CH2OH) and methanol (CH3OH); and alkanoic acids, e.g. ethanoic acid (CH3COOH) and methanoic acid, HCOOH exhibit hydrogen bonding.
The following is the order of strength of hydrogen bonding in the given compounds:
CH3COOH > H2O > CH3CH2OH
The above order is due to the different degree of hydrogen bonding per molecule in the compounds.
Unusual properties of compounds with hydrogen bonding:
Compounds with hydrogen bonding show unusual properties, such as: high boiling points- we would expect that boiling points should increase with molecular weight.
However, in comparing the boiling
points of H2O, HF and NH3 ,which exhibit hydrogen bonding with H2S, H2Se and AsH3
which do not exhibit hydrogen bonding but have greater molecular weight, it is found that H2O, HF and NH3 have boiling points unusually greater than the others.
Compounds with hydrogen bonding, in addition to unusual high boiling points also show unusual high melting points, heats of vaporization, heat of fusion, and viscosity. Their crystalline solids are usually very hard (e.g. ice – the extent of hydrogen bonding is greater than in water).
Unusual solubility – some compounds, containing oxygen, fluorine or nitrogen are unexpectedly soluble in hydrogen containing solvents due to hydrogen bonding. Examples include: the solubility of CH3CH2OH, HCOOH and NH3 in water.
|