|
Home
Shapes Of Simple Molecules
Molecules of different substances have been found to
possess some form of shapes. These shapes can also be a basis of studying and
understanding their properties.
Linear Molecules
The striking feature about linear molecules is that there is mutual repulsion about all the electron groups present, thereby having their atoms aligning on a straight line, with bond angle of 180o.
Examples of linear molecule include all diatomic
molecules, i.e.,
H2 → H — H
O2 → O — O
Cl2 → Cl — Cl
Also, certain polyatomic molecules are linear:
CO2 → O = C = O
Ethyne, C2H2 → H – C ≡ C – H
Non – linear Molecules
In non-linear molecules, the repulsion between the electron groups is not the same, it is found that repulsion between a lone pair and a bonding pair is greater than that between two bonding.
This is responsible for the bent structure of H2O molecule, and the triangular pyramidal or trigonal planar shape of NH3 molecule.
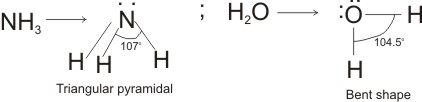
Note: the angle between two bond pairs in ammonia is larger (107o) than that of H2O molecule (104.5o). This is due to greater number of lone pairs on the oxygen of H2O, which exert greater repulsion on the bonding pairs.
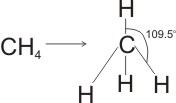
Tetrahedral Molecules
Tetrahedral molecules result when tetravalent atoms (e.g. carbon and silicon) undergo complete covalency with atoms of the same element. The four covalent bonds are distributed symmetrically in three dimensions, with the angle between any two bonding pairs being 109.5o.
Examples can be found in methane, CH4; silane, SiH4; and tetrachloromethane, CCl4
|