|
Home
Neils Bohr Atomic Model
In 1913, Neils Bohr put up a theory to primarily explain the existence of the hydrogen spectrum. The spectrum of hydrogen on a photographic film shows a series of narrow distinct lines of
colors.
Note: spectrum of hydrogen is a line spectrum and not a continuous spectrum which are obtained for elements with more than 1 electron.
Bohr put up the following postulates to explain the spectrum of hydrogen:
1. The electrons in an atom have only certain definite stationary states of motion, designated n, allowed to them.
2. Each of these
states (also called
shells or energy
levels) has a definite or fixed energy - i.e.,
the energy is quantized. The energy
increases outwards from the nucleus – the
states are usually numbered or lettered as
shown below.
3. The electron can continue to move in a particular orbit (usually the innermost, i.e. orbit of lowest energy) without the atom radiating energy – the electron is then said to be in the ground state.
4. When the atom gains energy, the electron may be raised to a higher orbit (or state) of greater energy – it is unstable, and is said to be excited. If the source of energy is removed, the electron falls back to the ground state and the fixed amount of energy, which was used in raising it to a higher level, is released as electromagnetic radiation of a definite
color or frequency.
5. The transition from one orbit to another occurs at once and not gradual.
6. Therefore, if several outer, higher energy levels are involved, an optical spectrum should be obtained, showing several lines at definite frequencies.
7. The amount of energy, given as hµ (h is the plank’s constant,
µ is the frequency) emitted, is equal to the difference of energy between the exited state and the ground state. For example, if an electron absorbs energy and is raised from a level
of energy E1 to another of E2, the energy
diagram
emitted when it falls back to E1 is
∆E = E2 – E1
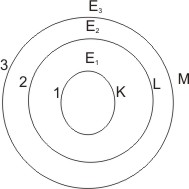
Explanation of the hydrogen spectrum – each distinct narrow line of
color represents fixed amount of energy (of a particular frequency) release as a result of the fall of the electron between any closest
two energy levels, as the electron falls
eventually to the ground level. Bohr’s theory
has not only explained perfectly the line
spectrum of hydrogen, but to some degree
the spectra of atoms with more than one
electron.
This theory, which suggests the
existence of energy levels, explains why an
atom does not collapse in spite of the
forces of attraction of the nucleus on the
electrons around it. Although not perfect,
Bohr’s atomic theory gave a refined
concept of the arrangement of electrons in
an atom. An improvement on the Bohr’s
theory is the modification of the quantum
theory suggests the presence of smaller
energy levels (sub-energy levels or sub-
shells) in each of the main energy levels.
These subshells consist of orbitals in which
electrons actually occupy. This explains the
continuous spectra obtained for polyelectronic systems (i.e., atoms with more than
one electron).
The structure of the atom as presently believed is that with a center (i.e., nucleus) where the protons and neutrons are concentrated – giving the mass of the atom. The electrons are arranged in specific energy levels (which are described by quantum numbers) around the nucleus.
These electrons are however not located in definite orbits in space as given by Bohr, but move around in an area which cannot be accurately determined with any certainty. The force of attraction between the protons and the electrons hold the atom together as a discrete particle. Also, the positive charge of the protons completely balances the negative charge of the electrons, making the atom to be neutral (without charge).
|