|
Home
Metallic Bonding
Metallic Bonding
is a special type of covalent bonding (different from the conventional covalent
bonding) that holds atoms of metals together. The atoms in a metal are closely packed in a crystal lattice. Depending on the type of crystal structure the metal has, each atom has eight neighbors (this is known as body centered cubic lattice) or twelve neighbors (this is either called a hexagonal or a cubic close- packed lattice).
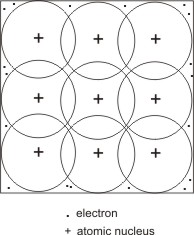
Metallic bonding is described thus: the valence electrons of each atom are loosely held to their nuclei, hence, they separate from individual nucleus to move at random through the crystal lattice. Positively charged metal ions are left, which tend to repel one another, but are held together by the moving electron cloud.
An explanation of this bonding is thus: each metal atom has more orbitals (such as 3d) available for bonding than there are bonding or valence electrons. Due to the atoms being closely packed, the valence electrons are not being limited to any specific bond orbitals between any two particular atoms (i.e. they are delocalized), but occupy one or more bonding orbitals (which are shared mutually by each of the neighbouring atoms).
Factors which affect metallic bonding:
1. The greater the number of valence electrons - the greater the number of valence electrons the greater the nuclear charge. This leads to greater strength of metallic bonding.
Example: comparing the strength, hardness and melting points of elements of period 4 – these properties increase from K (1 valence electron) to a maximum in chromium (6 valence electrons) – this is due to increase in metallic bonding. Hence, metallic bonding is strong in some metals,
example, iron and chromium, making them difficult to cut or shatter, but, it is weak in some other metals,
example, sodium and potassium which can easily be cut with a knife.
2. Nuclei size - the smaller the nuclei size the more the outer electrons are attracted and held, hence the greater the strength of metallic bonding. This also explains why the strength of metallic bonding increases from K to Cr in period 4.
Generally, the properties of metals, due to the presence of metallic bonding are:
1. Fairly high melting point.
2. Hard or soft depending on the degree of covalency.
3. Malleability and ductility – due to the flexibility of the direction of the mobile electrons.
4. Good electrical and thermal conductivity – due to the mobility of the electrons.
|