|
Home
Ideal Gas Equation
Ideal Gas Equation
Combining Boyle’s, Charles’ and
Avogadro’s laws, we obtain:
PV = nRT - this is known as equation of state of an ideal gas (or general gas law equation). n is the number of moles of the gas and R is the gas constant. P, V and T are the pressure, volume and the absolute temperature of the gas respectively.
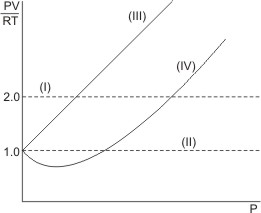
Plotting the graph of PV/RT
over pressure, we obtain the horizontal straight lines (see below) for idea or perfect gases.
Line (I) represents 2 moles of an ideal gas, while line (II) represents 1 mole of an ideal gas. Lines (III) and (IV) represent real gases – real gases deviate from ideal behaviors at high pressures and low temperatures.
Notice that at the limit of zero pressure, all gases show perfect or ideal behavior.
Calculation Based on Ideal Gas Equation
Calculate the volume occupied by 5.0 g of carbon monoxide gas at 25
oC and 0.97 4 atm pressure. (R = 0.082 liter-atm per mole per deg, C=12, O=16).
Solution:
T = (273 + 25) = 298 K, P = 0.974 atm
n = number of moles = mass/molar mass = 5.0 /28 = 0.18 mole
PV =nRT
V = nRT/P
= 0.18x0.082x298/0.974 = 4.5 liter (or dm3)
|

