|
Home
Examples of Electronic Configuration
Here are examples of electronic configuration of
atoms of different elements:
Hydrogen (1H) – contains one electron. This electron will be accommodated in the s orbital of K shell. Therefore, the accommodation of this electron is written as 1s1 . The number before the orbital indicates the main energy level the orbital is. The superscript is the number of electrons accommodated by the orbital.

Helium (2He) – contains two electrons. The first main energy level (K) is completely filled by the two electrons. The one s orbital of the shell is completely filled. Therefore, the configuration of the electrons is : 1s2.
Lithium (3Li) – contains three electrons. From the discussion above, the first main energy level (K) can take a maximum of two electrons, after which, the next shell (L), which can accommodate a maximum of eight electrons are filled. Therefore, to accommodate the three electrons of Li, two are taken into the one s orbital of K shell, while the remaining one electron is taken into the one s orbital of L shell.
Therefore, the configuration is written thus: 1s22s1.
Note: similar orbitals of different shells differ in energy. The lower the shell number, the lower the energies of it’s orbitals, hence the s orbital of the first shell is of lower energy than those of higher shells.
Boron (5B) – contains five electrons - two in the s orbital of the first shell (i.e. K), with the configuration 1s2. The three in the second shell (i.e. L) are distributed amongst its s and p orbitals, i.e., 2s22p1.
Notice that the s must be completely filled before the p can begin to fill. Altogether, the electronic configuration of boron is 1s22s22p1.
Neon (10Ne) – contains ten electrons – the first shell takes up two, and the second shell takes up the rest eight. The two in the first shell are taken up by its s orbital, i.e. 1s2. The eight in the second shell are distributed amongst its s and p orbitals, i.e., 2s2 2p6.
Altogether, the electronic configuration of neon is : 1s22s22p6. Notice that the electronic configuration of neon shows all the main energy levels (i.e., 1st and 2nd shells), and all the orbitals (s and p) to be completely filled with electrons.
This type of configuration confers on neon and such atoms with this type of configuration a perfect stability – the elements are not reactive and are regarded as noble gases.
Sodium (11Na) – contains eleven electrons. The configuration of neon above shows both the 1st and 2nd shells to be completely filled. Therefore, the eleventh electron (for Na) will be accommodated in the 3rd shell (in the s orbital of one of the subshells).
The electronic configuration of sodium is therefore: 1s22s22p63s1.
Argon (18Ar) – contains 18 electrons. Again, from the electronic configuration of neon, we need to account for another 8 electrons to state that of argon. These 8 electrons are to be accommodated in the 3rd shell, which can take a maximum of 18 electrons. The s and p orbitals of this shell accommodate these electrons, i.e., 3s23p6.
Therefore, the electronic configuration of argon is 1s22s22p63s23p6. Notice that this electronic configuration is similar to that of Ne above. The complete filling of the available orbitals confers on it a perfect stability.
Potassium (19K) – contains 19 electrons, just an electron more than argon. To account for the accommodation of this extra electron, we would have expected the electron to go into the 3d-orbital (since the 3rd shell can take a total of 18 electrons into it s, p and d orbitals). But this is not the case, as the extra electron goes into the s orbital of the next shell (I.e., the 4s). This is because the 4s orbital is closer to the nucleus than the 3d orbital, hence, it occupies a lower energy and therefore is more stable.
Thus, the electronic configuration of K is 1s22s22p63s23p64s1.
Calcium (20Ca) – 1s22s22p63s23p64s2.
Note: from the electronic configuration of an element, e.g.1s22s22p63s23p4, we can deduce
the following:
1. The atomic number of the element. The sum of the superscripts (number of electrons accommodated) by all the orbitals in an expressed electronic configuration equals the atomic number (number of protons) of the elements.
For example, the element above has a total of 2+2+6+2+4 = 16 electrons, hence the atomic number is 16.
2. The element. From the deduction of
atomic number, we then know the element.
For example, the above electronic configuration is for sulphur (whose atomic number is 16).
3. The class the element belongs. To know
the class (i.e., s, p, or d, etc) that the element
belongs, we look at the outermost orbital. For example,
the element whose electronic configuration
is given above is a p-block element because
its outermost orbital is 3p.
4. The group of the periodic table the
element belongs. This is same as the
number of electrons in the outermost shell.
For the above element, the configuration of
the outermost shell is 3s23p4. There are
2+4 = 6 electrons in the outermost shell,
hence the element is in group 6.
5. The period of the periodic table the
element belongs. This is same as the
outermost shell number. The above element
is in period 3 because its outermost shell is
the third shell.
6. The chemical properties of the
element. From the electronic configuration
of an element, we can deduce whether it can
easily lose electrons (metallic properties) or
gain electrons into its outermost shell (non
metallic properties). For example, the element with
the configuration 1s22s22p63s1 can easily
lose the one electron in its outermost shell
(3s1) while the element 1s22s22p5 can easily
gain one electron into its 2p orbitals to make
them complete.
The element with the
configuration 1s22s22p6 can neither lose
nor gain electrons into its outermost shell
because all available orbitals are completely
filled. Hence the element is inert or unreactive.
Also Note:
1. The electronic configuration of hydrogen
shows its only electron in the K shell, which has
one, or strictly speaking no subshells. Hence,
the electron is of a definite energy. This is why
the spectrum of hydrogen is a line spectrum.
2. The electronic configuration of atoms with
many electrons shows electrons in higher shells
(i.e., L and M). These shells are divided into subshells which are of different energies. Hence,
the electrons contained are of different energies.
This is why a continuous spectrum, which
indicates the presence of the subenergy levels
(i.e. subshells) is obtained, rather than a line
spectrum.
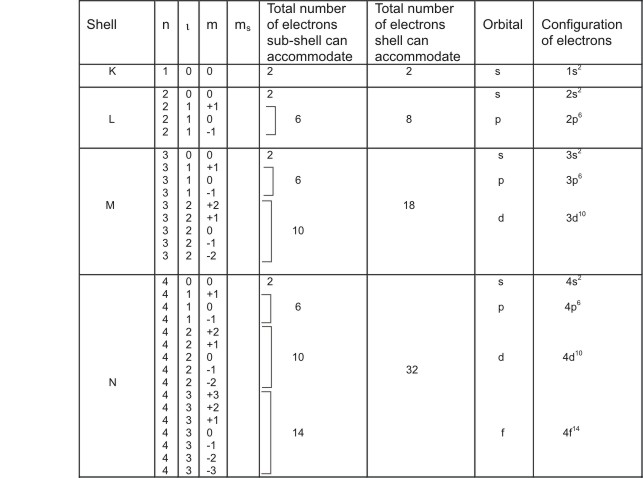
Electronic Configuration Table
The table below gives the summary of electronic
configuration in atoms - the arrangement of electrons in shells and subshells.
|