|
Home
The Discovery of Electrons
From series of experiments conducted by J.J. Thomson using the discharge tube (crook’s tube or cathode ray tube), he found that when a gas (e.g., neon, air, mercury
vapor, helium or any other less reactive gas) is placed in the tube at lower pressures and a high voltage applied, the gas is ionized and it conducts electricity, with the observation of a greenish glow at the cathode.
The glow consists of particles which were called cathode rays - because they were produced at the cathode.
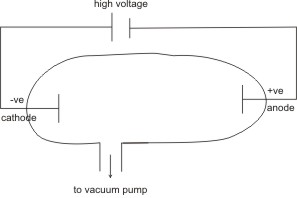
Note: - Gases conduct electricity at low pressure. At high pressures they don’t.
- The ionization of gases faults Daltons idea of atoms being the smallest and indestructible particles of matter.
The explanation of the above observation: at low pressures, and with the application of high voltage, gas molecules ionize, and since they are few, the distance between the ions is very great, hence the force of attraction between these ions is considerably low.
Therefore, the positive ions will cause a great deal of impact when they strike the cathode. This causes the emission of the greenish glow from the cathode.
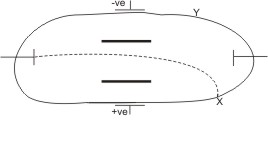
The nature of the cathode rays were examined and found to be the following:
- They travel in straight lines.
- They are deflected to the positive end of an electric or magnetic field, indicating them to be negatively charged particles.
- The mass of an electron is 0.000548 amu.
Cathode rays were found regardless of what gas is used in the discharge tube. For this, it was believed that they were not one particular type of electrified particles, but a fundamental particle of matter – they were later named electrons. J.J. Thomson went further to determine the charge to mass ratio (e/m) for the cathode rays. He hoped that this would help in identifying them either as ions or as some other charged fragment.
He found this ratio to be -1.76 x 108 coulombs per gram (the negative sign indicates electrons to be negatively charged particles).
Determination of Charge On An Electron by Millikan
The actual charge on an electron was found by R.A. Millikan (1907). He observed that in the presence of ionized air, tiny oil droplets would pick up an electric charge and become attracted to an electrode.
The amount of charge on individual droplet can be found from measurements of how fast the droplet falls to the plate by gravity (in the absence of an electric field), and how fast it rises (in the presence of an electric field).
He found that although the charge on different droplets were not the same, the total charge was always some integral multiple of the same unit of charge,
-1.6 x 10-19 coulombs. Hence, the smallest possible charge any one oil droplet can pick up is -1.6 x 10-19 coulomb - this is the charge on an electron.
|