|
Home
Citric Acid Hazards: Formula, Uses, Properties
This post provides detailed information about citric acid, its properties, formula, and uses, and the hazards commonly associated with it.
It provides useful ideas on how to safely handle citric acid to avoid exposure to its hazards, as well as what to do if having body contact with the chemical compound.
Please, continue reading to increase your knowledge about citric acid:
What is Citric Acid?
Citric acid is a chemical substance widely used as food additive. Naturally, it is derived from citrus fruits (orange, lemon, and lime) and other plants as well.
Many people consume different fruits every day, some don’t only consume but also use fruits to prepare homemade remedies.
Some of the common fruits are orange, lemon, and lime. These three fruits are known as the Citrus Fruits because they contain citric acid.
Citric acid is one of those compounds that contain carbon atoms (organic compound).
Citric acid has a lot of benefits and uses.
Citric Acid Formula and Structure
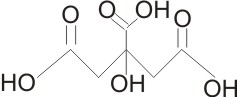
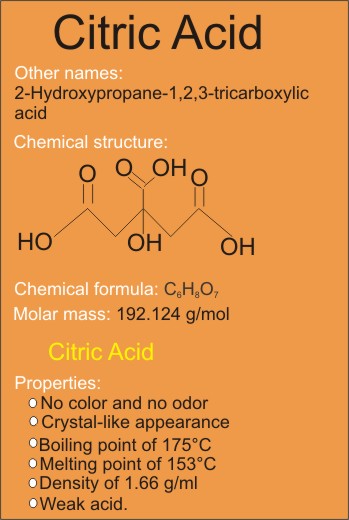
Citric acid, with IUPAC name 2-Hydroxypropane-1,2,3-tricarboxylic acid, is commonly identified with its chemical formula C6H8O7, which shows that a molecule of citric acid is made of six carbon, eight hydrogen, and seven oxygen atoms.
Citric Acid Structure
Common Citric Acid Hazards
Citric acid is a well-known and useful chemical. But it also comes with its own hazards and risks.
Unlike other chemicals, citric acid is not very fierce and harsh on the skin. It does not cause any serious irritation or burns on the skin.
With good gloves and protective clothing, your body is totally secured from this chemical.
Ingesting citric acid is not advisable at all.
It has the ability to hurt your throat and burn the walls of your stomach if a huge quantity is ingested.
Your eyes are not left out. In most cases, if citric acid enters the eyes it causes serious discomfort, itching, and some pains.
 Citric Acid Safety Handling Procedure Citric Acid Safety Handling Procedure
When handling citric acid, there things you should do to protect yourself from exposures:
• Before handling this chemical, put on protective clothing that keeps you safe against the chemical
• Always make sure your eyes are protected with the recommended goggles that can withstand chemicals
• Avoid ingesting citric acid when working with it (whether small or large quantity).
To guide against tooth corrosion, avoid excess consumption of citric fruits and drinks that contain citric acid.
But if you insist on consuming citric acidic products and fruits, make use of straws. This will help you consume the drink without allowing them to touch your teeth.
Citric Acid Safety Storage Procedure
The storage method for citric acid is totally different from other chemicals. There are specific procedures to follow when storing citric acid, to ensure the chemical is well preserved.
Citric acid demands a very safe storage method. You should know that every chemical requires a special type of tank or container for its storage because of its unique properties.
Consider the following when storing citric acid:
• Strength of the storage tank: Examine the tank and make sure it is durable enough to hold the chemical for as long as possible
• Leakage: A leaking tank is not appropriate to store chemicals. It will also cost you money to fix the storage environment polluted with the chemical
• Guide against corrosion: Make sure you use a storage tank that is compatible with citric acid to prevent corrosion. Using a tank that does not lead to corrosion.
Citric Acid Safety Disposal Procedures
The best way to dispose citric acid is by neutralizing it first, so that it can be safe for disposal.
You can neutralize it by mixing it with water and a baking soda.
After adding these, the citric acid is very safe to pour down the drain.
How to Handle Exposures to Citric Acid
Apply these first aid measures if you get exposed to citric acid:
If it touches the skin, rinse it off with plenty of water; pull off the clothes affected by the chemical.
Make sure you rinse your eyes if citric acid enters them. You can contact the nearest medical facility if you are not satisfied with the first aid method.
Additional Citric Acid Information
Here is additional information about citric acid to help you learn more about the chemical compound:
Citric Acid Properties (Physical and Chemical)
Citric acid has the following properties:
• No color and no odor
• Crystal-like appearance
• Molar mass of 192.124 g/mol
• Boiling point of 175 degree Celsius
• Melting point of 153 degree Celsius
• Unique solid density of 1.66 g/mL.
Citric acid has been discovered by scientist to be one of those weak organic compounds. It is water free (popularly known as anhydrous), and this only happens when citric acid is heated to a specific degree Celsius (usually 78 degree Celsius).
Citric Acid Preparation
Citric acid can be derived from fruits like orange, lemon, and lime (they are also known as citrus fruits) by extracting it from the fruits.
Another preparation method is by fermenting various carbohydrates like corn and sugar cane.
Citric Acid Uses
Citric acid plays a very important role in foods. Many industries use it as a food additive; it also serves as a preservative in food (especially in processed foods).
|